Basic Idea-
A crystal is a substance which is formed by repetition of 3-d identical units,where a unit may contain one or more atoms.
Some crystallographic terms-
lattice-
is a periodic array of geometric points arranged in space such that each point is identical to surrounding.
Basis-
is obtained by placing an atom or group of atoms at each lattice point in regular manner.
lattice+basis=crystal.
unit cell-
this is the region of the crystal defined by basis vectors a,b,c.
lattice constant-
a,b,c , alpha , beta , gama are lattice constants.
Bravais lattice-
there are only fourteen ways of arrangements of points about given point
some basic structures-
cubic lattice-
there are 3 basic cubic lattice
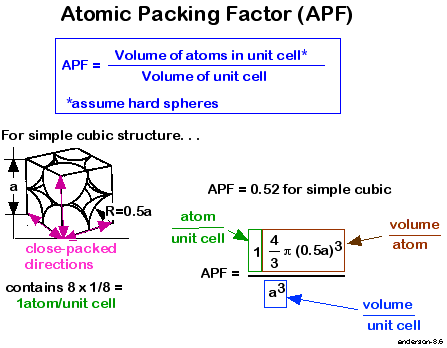
simple cubic- no of atoms=8*1/8=1example-polonium
body centered cubic=8*1/8+1=2 example-Ba,Fe,K,Li
face centered cubic=8*1/8+6*1/2=4 example-Al,Ca,Cu,Ni
packing fraction-
it is the ratio of volume of the sphere in unit cell to the volume of unit cell.
Miller indices-the orientation of a crystal plane within the crystal can be determined by any three nonlinear points in the plane.
if each of the points lies on crystal axis, the position and the orientation of the plane may be obtained by specifying the location on the axis in terms of lattice constants on basis vectors